Many sick Nigerians are dying by installments on daily basis; this is not always because they don’t have the money to purchase the drugs to heal their ills; they are buying what may quickly kill them as drugs and unknowingly, they pop counterfeit and substandard pills paraded as potent drugs in the market. Like it or hate it: Fake drugs can damage your health, it kills you slowly even before your death.
Opeyemi Jamiu has gulped poisoned pills and he’s about to pop his clogs, the flooding saliva of his mouth has deserted him and his throat totally dry. He’s heavily gasping for breath, suffering from severe dehydration; if he spends more time without proper medications, he’ll give up the ghost; but professional medical practitioners in his community clinic have come to his aid; the doctors confirm that he has taken some toxic tablets – killer drugs capable of damaging his health. Carelessly, while returning from school, feeling an unbearable headache; Jamiu decides to visit one of the medicine stores at the back of his school, asking the patent drug seller to give him a drug to cure his ailment. “I can’t even remember the name of the drug – he just cut it for me and I left for home,” he recounts.
Funnily enough, he gets home to find himself in the land of the dreamers – in deep sleep – and wakes up the next morning with a more chronic headache, worst than his last night’s health ordeal; he pops the drugs bought yesterday before leaving for his cousin’s place, on a Friday morning.
“When I got to my cousin’s place, we were discussing and suddenly, I just noticed I could not feel any saliva in my throat again. Immediately I noticed that, I rushed to the kitchen myself and took some palm oil; I felt a little bit okay but some minutes later, it occurred again. I told my cousin that I could not understand how I was feeling, so we went to one health centre nearby – Lamodi Health Centre, Offa – a community clinic in the area, where they gave me injections. They began to ask me some questions, trying to know what I ate, and that was the last thing I saw,” he says. “When I woke up, I was a little bit okay; the doctor asked if I had used anything before coming to the hospital and I told him that I used some drugs; he confirmed that I have popped poisoned pills.”
Many sick Nigerians are dying by installments on daily basis; this is not always because they don’t have the money to purchase the drugs to heal their ills; they are buying what may quickly kill them as drugs and unknowingly, they pop counterfeit and substandard pills paraded as potent drugs in the market. Like it or hate it: Fake drugs can damage your health, it kills you slowly even before your death. A study by the World Health Organisation (WHO) unveils that those counterfeiters have massively killed millions of people in developing countries (such as Nigeria). “An estimated 1 in 10 medical products circulating in low-and middle-income countries is either substandard or falsified,” a research by WHO has revealed.
Meanwhile, any drug could be a killer, no matter how good it appears, if the formulation of a drug is poorly made or criminally produced, it is either fake or substandard. Drug counterfeiters are mere profit makers, they are careless about the public health but concerned about the fat gains made from fake medicinal products that are dangerous for human consumptions.
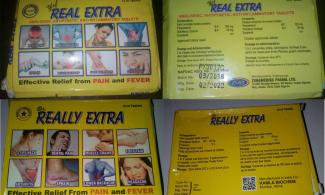
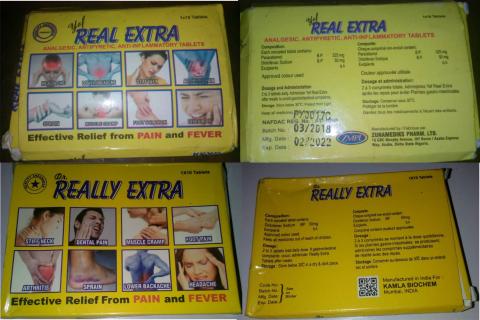
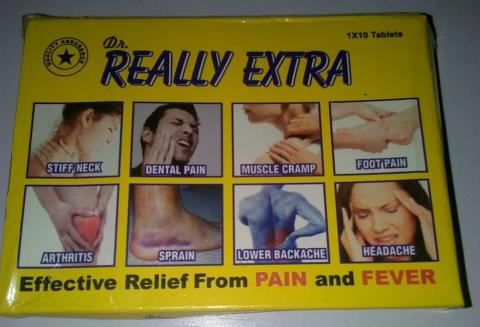
For instance, ‘Dr. Really Extra’ tablet brand is fancifully appealing to the layman’s eyes; the glittery, yellowish pills boast to bear active diclofenac potassium that ‘kills’ all kinds of pains – as boldly written on the pack – but be careful of the eye-teasing drug! Veteran drug analysts in the College of Medicine, University of Lagos, vouch that it’s criminally made and should be eliminated totally from drug marts. Its lookalike is ‘Yef Real Extra’ tablet, which seems to be the original brand of ‘Dr. Really Extra’. It bears the NAFDAC no: A11-0298 but fails the test of the active ingredient woefully. A laboratory test has confirmed that the drug is substandard and not safe for human consumption. These are just a few examples of the poorly and criminally-made drugs in the country. A quest to unravel the business of fake drugs pushed this reporter out on a Monday morning.
Analytical Expedition into the Realm of Drug Counterfeiting
“Good morning ladies and gentlemen! As we are all going out today to find our daily bread, may we not get into trouble in the course of our struggles in Jesus name,” a short, fair man with a clerical look prays in a sonorous voice, to which there is a chorused response represented in shouts of “Amen” from the crowd, in the 18-passenger bus. It is barely 10.00 am; the slight sunny weather is not too hot, for a heavy downpour has just wrapped up.
The passengers have just struggled to find their ways into the Orile-Oshodi bus, after a tense delay at the vehicle terminus, amidst the give-me-way-if-you-can’t-go cacophony in Lagos.
“My name remains unchanged. I am Obinna. I know that many of you are stressed up this morning. Some of you already have headache even before getting to your places of work. The Bible says: ‘Dear friend, I pray that you may enjoy good health and that all may go well with you even, as your soul is getting along well.’ Ladies and gentlemen, I bring to you this morning ‘Dr. Really Extra’, a pain killer that threatens all sorts of pains in the body. You’re still under 30 but you can’t stand for one hour without crying of backache. My brother, my sister, you wake up in the morning, go to work and return home, having had a troublesome day; you need this drug to kill the pains that give you terrible nightmares every day.”
“Health is wealth,” he continues, “if you’re having a stiff neck, hot headache, foot pain … whatever kinds of pains, don’t keep silent. ‘Dr. Really Extra’ is here to tackle them all. If you know, you know; those who know me know I don’t sell trash. All the way from India, ‘Dr. Really Extra’ is just N100. Buy and don’t forget to get for your relatives. The Bible says: ‘Foreigners lose heart and come trembling from their fortifications’.”
“O yes!” A woman in the bus exclaims, as the man solicits people’s patronage. “With ‘Dr. Really Extra’, every evil spirit of pain in your body shall be killed in Jesus name.” “Amen,” passengers in the bus echo again and a slight scramble begins as they order for the drug – it is a lucrative Monday morning for the drug counterfeiting trader.
This reporter purchases some packs of the drug; a critical look at it gives room to doubt whether the drug is actually genuine as claimed. The label of the yellow packet of the said ‘Dr. Really Extra’ has no NAFDAC number, no batch number, no expiry date, not even on the blister (as the labels indicate). When asked whether the aforementioned is not a reason to doubt the potency of the drug, Obinna responds in pidgin: “No worry. That one no mean anything. The important thing na make the drug work well well.”
However, it indeed means a lot; the World Health Organisation (WHO) refers to such drugs as“unregistered/unlicensed medical products that have not undergone evaluation and/or approval by the National or Regional Regulatory Authority for the market in which they are marketed/distributed or used, subject to permitted conditions under national or regional regulation and legislation.”
The body saddled with the responsibility of regulating the production of food and medicinal products in Nigeria, the National Agency for Food and Drugs Administration and Council (NAFDAC), strongly prohibits the importation, exportation, buying and selling of any medicinal products that are either counterfeit/fake or substandard. WHO has defined counterfeit medicine as “one which is deliberately and fraudulently mislabelled with respect to identity or source.” This, in fact, is an apt description of ‘Dr. Really Extra,’ which is commonly sold to common peoples in Nigeria.
The Drug Analysis

“Mtchew,” Mr. Olajide Mustapha hisses disappointedly as he stares scrupulously at ‘Dr. Really Extra’ brand pack. He inclines his head left and right; a wrinkle of anger is tentatively plastered on his face. “Those Indian people,” he quips and gloves his hands to begin the test of active ingredients for the selected drugs – this takes place inside the drug analysis laboratory at the College of Medicine, University of Lagos. Mr. Peter Ojobor, the Assistant Chief Technologist, leads the drug analysis, aided by Mr. Olajide Mustapha.
Five pills of ‘Dr. Really Extra’ are later placed in the Denver Instrument (Analytical Weighing Balance) to weigh the substances; the pills are triturated, tooling the porcelain mortar and its pestle. The ground substance is then wrapped up to get it weighed again in the Analytical Weighing Balance. Mr. Ojobor puts the powdered pills in the sample bottles. He uses the sonicator to give the drug an ultrasonic bath, so as to enhance the solution of the analyte (drug substance). The next step is to mix and shake the analytes to ensure the solubility and subject them to the syringe filter to round off the analysis and await the final results.
Get it right: ‘Dr. Really Exra’ tablet is not the only diclofenac drug subjected to laboratory test by this reporter. Having x-rayed the open drug markets, buying drugs randomly, from chemists and petty drug peddlers in commercial buses and on the streets – in the hearts of Lagos, Oyo, Sokoto and Niger states, respectively – four affordable and common pain killer drugs (diclofenac) are sampled and subjected to test in the laboratory. The sampled drugs are: ‘Dr. Really Extra, Yef Real Extra, Masterfen 50g and Diclosuit.’ The same methods of drug analysis stated above are used for all the drugs.
‘The Criminally Produced Dr. Really Extra’
According to the certificate of the drug analysis issued to this reporter, dated April 12, 2019, ‘Dr. Really Extra’, which is a“sachet yellow caplet scored on one side and plane on the reverse.” It is however clearly asserted that “the product passed the assay of active ingredient (Diclofenac) but the regulatory details are not satisfactory.”
Mr. Ojobor states that ‘Dr. Really Extra’ is an illegal brand medicinal product which violates NAFDAC’s regulatory laws. “Even though the formulation is okay, there are drug regulatory laws in the country. For instance, this one contains the contents it claims, but if they produce another one, the content may be too high or too low. If the content is too high, it causes toxins and so, if it causes damage to the body, the regulatory body cannot trace the source. From what I have seen here, that drug is criminally produced; there is no address of where it is produced; it is not registered; it is not dated. So, it is a criminal brand.”
Meanwhile, NAFDAC’s Food, Drug and Related Products (Registration) Act Cap F.33 wholly prohibits the manufacture, sale, distribution and advertisement of unregistered food, drugs, drug products, cosmetics, medical devices or water. The Law also regulates the manufacture, importation, exportation, advertisement, sale or distribution of processed food, drugs, medical devices, chemicals and bottled water. Subsection 1 of the said act decrees that “no processed food, drug, drug product, cosmetic, medical device or water shall be manufactured, imported, exported, advertised, sold or distributed in Nigeria unless it has been registered in accordance with the provisions of this Act or regulations made under it.”
Poorly Produced But NAFDAC Approved…
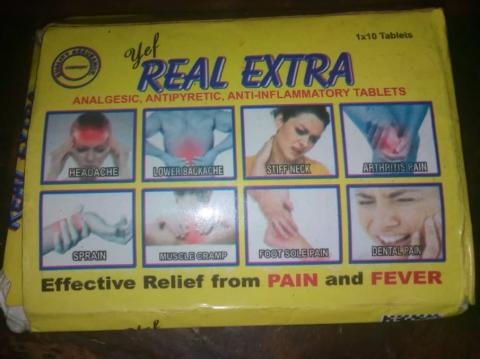
Only a critical look will quickly fathom that ‘Dr. Really Extra’ is a copy of ‘Yef Real Extra’; of course, the drug’s brand name of the later is modified to make the former. Yet, the ‘Yef Real Extra’ is not ‘real’; when checked on the NAFDAC website, it has a documented database with the regulatory body, which means it is duly registered and approved by the agency, unlike its lookalike – Dr. Really Extra – which has no database with NAFDAC.
Nevertheless, ‘Yef Real Extra’ is poorly produced but NAFDAC approved; it fails the test of its diclofenac active ingredient – the content of the drug is said to be below the 90-110% limit of specification. What this implies is that the ‘pain killer drug’ is substandard and not safe for use. “Scored on one side and plane on the reverse,” is stated on the certificate of analysis dated April 4, 2019.
Substandard drugs are – according to WHO – “authorised medical products that fail to meet either their quality standards or specifications, or both,” just like ‘Yef Real Extra’. “Substandard and falsified medical products are often produced in very poor and unhygienic conditions by unqualified personnel, and contain unknown impurities and are sometimes contaminated with bacteria,” WHO states.
Exploring the health implication of the substandard drug, Mr. Ojobor asserts that “the efficacy (of the drug) will be reduced because it is not up to the 50mg it claims; of course the formulation must contain 50mg for it to have energetic effects. But if it is less than that, the effect will also be reduced. This is for pain relief, so the extent to which it will relieve the pain will not be adequate enough – the person will feel relieved but not as much as it will be, if it were to be a full dose.”
The question is: How did substandard ‘Yef Really Extra’ caplet get approved by NAFDAC?
Mr. Chukwuebuka Ejiofor, a pharmacist at Glory Land Pharmacy in Lagos has an idea of what could have gone wrong, which brings up the question of the approval of the substandard drug by NAFDAC.
“I have a friend who had internship with NAFDAC; there are some cases when most of these drugs don’t pass the required test and the ‘Ogas at the top’ will make you certify those drugs. So, even in our regulatory bodies, we still have corruption. For example, a tablet does not contain its Paracetamol 500mg and when assayed, it contained 450 and your ‘Oga’ says you should certify such product and issue it NAFDAC number; obviously that is corruption in the regulatory system,” says Mr. Ejiofor, adding that “sometimes it could be lack of required facilities.” “Ideally, for example, for you to import a drug, NAFDAC is supposed to travel outside the country to inspect the pack in question. Even when you’re manufacturing in Nigeria, they should look from their offices to inspect.”
The pharmacist also notes that “sometimes logistics issues come in … this is Nigeria for you. They are under-staffed; they cannot cover everywhere. It takes four to five months for NAFDAC to pass a particular drug and issue number. In Nigeria, they have a lab in Kaduna; they have a lab in Lagos and in one other place, serving the whole of the nation. So if the assay is not done in Lagos, it is pushed to Kaduna. How can they have just two assay outlets, serving 36 states?”
Notwithstanding, section 4 (1) of the NAFDAC Act states that: “The Agency may suspend or cancel the registration of a processed food, drug, drug product, cosmetic or medical device if (a) the grounds on which the processed food, drug, drug product, cosmetic or medical device was registered were later found to be false or incomplete; or (b) the circumstances under which the processed food, drug, drug product, cosmetic or medical device was registered no longer exist; or (c) any of the conditions under which the processed food, drug, drug product, cosmetic or medical device was registered has been contravened; or (d) the standard of quality, safety or efficacy as prescribed in the documentation for registration is not being complied with; or (e) the premises in which the processed food, drug, drug product, cosmetic or medical device or part thereof is manufactured, assembled or stored by or on behalf of the holder of the certificate of registration are unsuitable for the manufacturing, assembling or storage of the processed food, drug, drug product, cosmetic or medical device.”
By implication, as a drug regulatory body, NAFDAC has every right to withdraw the approval given to the drug, ‘Yef Real Extra’ for failing the assay of active ingredients, woefully.
Two Drugs Stand Out

Unlike the laboratory proven substandard ‘Yef Real Extra’ and its unlicensed copy –‘Dr. R eally Extra’– the other two drugs, which are subjected to both physical and laboratory tests stand out. The results of the drug analysis clearly show that both ‘Diclosuit’ and ‘Masterfen 50’ tablets pass the assay of active ingredients and abide by the drug regulatory laws, accordingly. ‘Masterfen 50’, with a sachet of pink caplets, which bears the NAFDAC no: A4-8180 is distinctively described to have passed the assay of active ingredient by 104.85% of labeled Diclofenac. The analysis shows that “the product is satisfactory and safe for use.” Diclosuit, an off-white tablet with blue, yellow and pink tinge, with NAFDAC no: B4-6742 also passes the assay of active ingredient with 110.62% of labeled claim for Diclofenac. It is said to also be satisfactory and safe for use.
Fake Drugs Kill Faster Than Disease!
He’s been apprehended by an infection that troubles his reproductory organ. He is in severe pain and if he dares waste more time before seeing his doctor, he may wake up to tell sad health stories that touch. He quickly consults his doctor and the doctor says he has been gripped by Epididymitis – a critical disease usually caused by bacterial infection. His own name is Mustapha Munir and he lives in Kaduna. He tellingly carefully follows the doctor’s prescriptions by going to purchase the drugs in a pharmacy, only for his condition to get more and more critical. Guess right: Munir has bought a fake/counterfeit drug and his condition gets worse, until he’s asked to change his pharmacy.
“I was having this serious problem called Epididymitis and the doctor prescribed a drug for me. It's actually an infection that affects person's reproductory organ. Following the prescription that he (the doctor) gave me, I got the medicine from a pharmacy. I was taking them (the antibiotic drugs) just the way they were prescribed to me, but the skin of that drug started getting void.
“We realised that it was actually fake drug that was sold to us and when I changed the pharmacy, the pain went off in few days,” Munir narrates. But then, before gaining back his good health, he has had serious pain, and felt like vomiting. “I kept feeling nauseous and dehydrated,” he says.
He nevertheless lamentably frowns the high level of fake drugs paraded in the society of nowadays, stressing that “fake drugs kill people faster than disease itself.” With a disease, we can live and be fine but once one starts taking fake drug, one may quickly die. He then wonders whether we are really fighting this war against fake drug, when humanity is nothing. “People would just keep dying and nobody tends to care about it,” he laments.
Munir however counsels that “awareness has to be created for people to fight back against drug counterfeiters.” “Once the awareness is being created,” he admonishes, “the institution that is supposed to make it their work, educating the people against fake drug would be able to find the moral in people’s depression by doing what the people want them to do.
“When they work, fake drug sellers would actually be eradicated. To eradicate fake drug, the people have to rise and the institution has to actually reform themselves, their activities and the system itself,” he says.
He also advices “victims of fake drugs in Nigeria to always be conscious,” noting that “ whenever they are buying drugs, they should verify and ensure that the drugs they are buying are authentic. They should spend quality money on quality drug. “If you are to get it at an expensive price, but knowing it's of quality, buy it.” He never forgets to remind victims that “whenever they find any kind of fake drugs within the society, they should report to the authority in charge of fake drugs.”
Curing Chronic Headache with Chalky Paracetamol

Amidst the rabble-rousing Kano motor park, Mrs. Linda is imprecated by a severe headache; he’s got no choice than to patronise one of the road-side-drug-peddlers in Kano, with the hope of getting a Paracetamol to cure her worrisome headache. Disappointedly, mere powdered chalk has been masqueraded as a Paracetamol tablet – she has purchased aggravating chalky pills.
“I was in Kano, at a motor-park, on my way back to Kaduna and I wanted to buy medicine because I had a headache and I called this road-side-peddler – you know in the park there are always so many of them walking around – so, I bought a Paracetamol from one of them. But surprisingly when I opened the medicine, it was powdering. I tasted it and it was actually chalk,” she says.
“I was furious and told others about the travail – they now told me that there were some factories that make these drugs in Kano and some other places. They deliberately package it and sell to unsuspecting buyers. That was when I realised that I should stop buying drugs from drug peddlers and that was how I knew some fake drugs especially in Kano.”
‘… Analgin Almost Paralised my Left Leg’
If not for professional medical intervention, Lukman , a resident of Ijora, Lagos would have had a paralised left leg by now. On a Tuesday morning, Lukman wakes up feeling feverish and unsettled; he uses several pills but none of them seems efficacious and so, he decides to call on a local nurse for treatment.
“The first thing I noticed while checking the drugs that was to be given to me by this nurse was that, there was an injection called Analgin, which did not even have a NAFDAC number,” says Lukman, narrating his ordeal. “I challenged the nurse; I told her that the injection did not have a NAFDAC number but she told me not to worry about that and I kept quiet when she told me not to teach her her job.”
Afterwards, the nurse injects the contraband analgin into Lukman’s body, through his ass vein, only for him to remain stiff, with a knocked knee. “For minutes, I could neither move forward nor backward. My left knee was knocked and my leg was also stiff. I cried.”
The nurse is dumfounded – too confused to know what to do. Lukman’s guardian quickly rushes him to a nearby private hospital – Jim Sam, located at Gasikiya Road, Ijora-Badia, in Lagos. “They treated me and told me never to patronise such nurse again. If not for the doctors in the hospital, that analgin almost paralised my left leg.”
Before now, Analgin injection used to be highly recommended as a pain killer and fever fighter. But in 2005, the National Agency for Food and Drug Administration and Control (NAFDAC ) banned the drug from circulation following “the discovery of two major cases of severe Adverse Drug Reactions (ADRs) involving two secondary school students at the Federal Government Girls' College, Ibuzor, Delta State. One of the students, a 14-year-old Miss Uju Okwusiogu, suffered severe tissue damage from the upper hip down to the back knee, while the other a 12-year-old girl, died following severe Toxic Epidermal Necrolysis (TEN) after being injected with dipyrone known as metamizole in generic terms.”
When asked by Lukman why she used the banned analgin on him, “she said that was not her first time of using alnalgin for her sick patient. She also said that she never thought it was going to happen that way.”
Pharmacovigilance
It is essential for drug regulatory bodies such as NAFDAC to invigilate thoroughly, the importation of drugs, examine the drugs before they find their ways into the open markets for human consumption and even after the they reach the markets (and pharmacies) – this is exactly what is known as ‘pharmacovigilance’.
However, a top NAFDAC official who prefers not to be named because he is not authorised to speak to journalists explains in details how the drug regulatory agency has been vigilant to ensure the safety of the drugs sold in Nigeria. “There is what we call drug registration number and drug compliance number. We ensure safety, efficacy and quality of the product. After we check and before we give NAFDAC approval, we have to look at the critical company of that product. We have to inspect where you are producing the drug, the process from the formulation to the finishing. The active ingredients, the source of formulation and whether you follow the right process or not.
“After meeting all the specified process, reaching the final stage, we also sample. When we sample, we will now take the product and check the claim of the manufacturer. In drugs, there is verification of active ingredient, we do potency, and we also check dissolution,” he says.
While stressing that any medicinal product without NAFDAC number should be regarded as counterfeit and be avoided, he explains that some drugs may still fail the assay of active ingredient when they are poorly stored.
“You know, these things that they use in making the drugs are scientific formation; they can easily degraded by either radiation or heat. There is something we call stability of a drug, if the drug is not stable. When I talk of stability, I talk of the storage condition of that drug, mode of transformation of that drug, and dispose of that drug. If it doesn't follow the due process, and at the end it reaches the consumer, they may not get the actual ingredients they are suppose to meet,” he remarks.
He also clarifies that “not less that 75 percent of the tablet should release into the body system. If any tablet releases lower than that, that tablet has failed quality control check. And that tablet can be called counterfeit or substandard. Dissolution will give the rate of drug release into the body system. Assay is just to quantify the percentage of active ingredients in that product.
“You may see some fine drugs with good packages but the active ingredient is not there. We have some machines that detect that. We make sure that all the anti-malarial drugs have our scratch cards on them. We collaborate with the entire network provider to authenticate the product passed by NAFDAC. But here in Africa some are mad in making money; some fake NAFDAC numbers,” he says, lamentably.
While the role of NAFDAC – as far as drug regulation is concerned – is to regulate and control the manufacture, importation, exportation, advertisement, distribution, sales and use of drug, the Pharmacists Council of Nigeria (PCN) is saddled with the responsibility of regulating and controlling the education, training and practice of pharmacy in the country. However, on its side, PCN has beamed silence, when contacted to explore the possible pharmaceutical blues that fumes the scourge of fake drugs in Nigeria and activities of the agency to curb the menace.
On May 21, 2019, an information seeking letter was written to the headquarters of the agency in Abuja, but as at the time of writing this report, this reporter has got no feedback; neither has he been granted an interview, as requested.
Before You Pop That Drug…
Perhaps you are a bit under the weather, gripped by acute headache, severe stomach disorder or even fatal fever, beware and be aware of certain things you may have to consider, before you pop that drug.
NAFDAC's Director of Public Affairs Dr. Jimoh Abubakar, warns Nigerians from buying drugs from sourceless counterfeiters, stressing that “the problem of drug counterfeiting is quite overwhelming; we are talking of a population over two hundred million (in Nigeria) and we have (in NAFDAC) a population of staff that is not more than two thousand five hundred, policing that Nigeria large population."
“But then, we have involved a creative way of doing this job so that we can stay ahead of the counterfeiters. And one of the strategies we have leveraged on is consistent public enlightenment campaigns – enlightenment campaigns in the sense that, instead of jumping here and there with limited staff strength, we try to educate the citizens in the right direction.
“And we have always been saying; when you see a product that does not have NAFDAC registration number, avoid it, because you cannot vouch for what is inside. Even if it has effect of high qualities, it means the manufacturer has something to hide. Even when the number is there, look out for other things – the batch number, the manufacturer address and other things.”
This report was done with support from Ford Foundation and the International Centre for Investigative Reporting, ICIR.